Inhaltsverzeichnis
Werbung
Verfügbare Sprachen
Verfügbare Sprachen
en
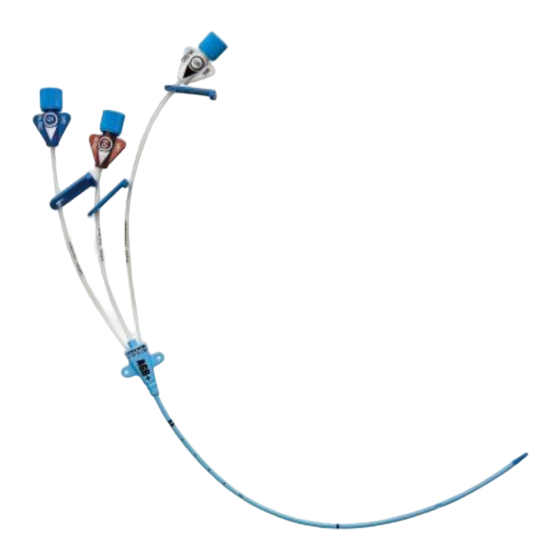
Pressure Injectable Central Venous
Catheter (CVC) Product
Rx only.
Indications for Use:
The Arrow catheter is indicated to permit short-term (< 30 day) central venous access for
the treatment of diseases or conditions requiring central venous access, including, but not
limited to the following:
•
Lack of usable peripheral IV sites
•
Central venous pressure monitoring
•
Total parenteral nutrition (TPN)
•
Infusions of fluids, medications, or chemotherapy
•
Frequent blood sampling or receiving blood transfusions/blood products
•
Injection of contrast media
When used for pressure injection of contrast media, do not exceed the maximum indicated
flow rate for each catheter lumen. The maximum pressure of power injector equipment
used with the pressure injectable CVC may not exceed 400 psi.
Contraindications:
None known.
Clinical Benefits to be Expected:
The ability to gain access to the central circulation system through a single puncture site for
applications that include fluid infusion, blood sampling, medication administration, central
venous monitoring, and the ability to inject contrast media.
General Warnings and Precautions
Warnings:
1. Sterile, Single use: Do not reuse, reprocess or resterilize.
Reuse of device creates a potential risk of serious injury
and/or infection which may lead to death. Reprocessing of
medical devices intended for single use only may result in
degraded performance or a loss of functionality.
2. Read all package insert warnings, precautions and
instructions prior to use. Failure to do so may result in severe
patient injury or death.
3. Do not place/advance catheter into or allow it to remain in
the right atrium or right ventricle. The catheter tip should be
advanced into the lower 1/3 of the Superior Vena Cava.
For femoral vein approach, catheter should be advanced into
vessel so catheter tip lies parallel to vessel wall and does not
enter right atrium.
Catheter tip location should be confirmed according to
institutional policy and procedure.
4. Clinicians must be aware of potential entrapment of
the guidewire by any implanted device in circulatory
system. It is recommended that if patient has a circulatory
system implant, catheter procedure be done under direct
visualization to reduce risk of guidewire entrapment.
5. Do not use excessive force when introducing guidewire or
tissue dilator as this can lead to vessel perforation, bleeding,
or component damage.
6. Passage of guidewire into the right heart can cause
dysrhythmias, right bundle branch block, and a perforation
of vessel, atrial or ventricular wall.
7. Do not apply excessive force in placing or removing catheter
or guidewire. Excessive force can cause component damage
or breakage. If damage is suspected or withdrawal cannot be
easily accomplished, radiographic visualization should be
obtained and further consultation requested.
8. Using catheters not indicated for pressure injection for such
applications can result in inter-lumen crossover or rupture
with potential for injury.
9. Do not secure, staple and/or suture directly to outside
diameter of catheter body or extension lines to reduce risk of
cutting or damaging the catheter or impeding catheter flow.
Secure only at indicated stabilization locations.
10. Air embolism can occur if air is allowed to enter a central
venous access device or vein. Do not leave open needles or
uncapped, unclamped catheters in central venous puncture
site. Use only securely tightened Luer-Lock connections
with any central venous access device to guard against
inadvertent disconnection.
11. Clinicians should be aware that slide clamps may be
inadvertently removed.
12. Clinicians must be aware of complications/undesirable side-
effects associated with central venous catheters including,
but not limited to:
•
cardiac tamponade
secondary to vessel,
atrial, or ventricular
perforation
•
pleural (i.e.,
pneumothorax) and
mediastinal injuries
•
air embolism
•
catheter embolism
•
catheter occlusion
•
thoracic duct laceration
•
bacteremia
•
septicemia
Precautions:
1. Do not alter the catheter, guidewire or any other kit/set
component during insertion, use or removal.
2. Procedure must be performed by trained personnel well
versed in anatomical landmarks, safe technique and
potential complications.
3. Use standard precautions and follow institutional policies for
all procedures including safe disposal of devices.
4. Some disinfectants used at catheter insertion site contain
solvents which can weaken the catheter material. Alcohol,
acetone, and polyethylene glycol can weaken the structure
of polyurethane materials. These agents may also weaken the
adhesive bond between catheter stabilization device and skin.
•
Do not use acetone on catheter surface.
1 1
•
thrombosis
•
inadvertent arterial
puncture
•
nerve injury
•
hematoma
•
hemorrhage
•
fibrin sheath formation
•
exit site infection
•
vessel erosion
•
catheter tip malposition
•
dysrhythmias
•
extravasation
Werbung
Inhaltsverzeichnis

Inhaltszusammenfassung für Teleflex ARROW ZVK
- Seite 1 Pressure Injectable Central Venous Catheter (CVC) Product Rx only. 7. Do not apply excessive force in placing or removing catheter Indications for Use: or guidewire. Excessive force can cause component damage The Arrow catheter is indicated to permit short-term (< 30 day) central venous access for or breakage.
- Seite 2 • Do not use alcohol to soak catheter surface or allow Gain Initial Venous Access: alcohol to dwell in a catheter lumen to restore catheter Echogenic Needle (where provided): patency or as an infection prevention measure. An echogenic needle is used to allow access to the vascular system for the introduction of a •...
- Seite 3 Advance Catheter: 16. Thread tip of catheter over guidewire. Sufficient guidewire length must remain exposed at hub end of catheter to maintain a firm grip on guidewire. 17. Grasping near skin, advance catheter into vein with slight twisting motion. Warning: Do not attach catheter clamp and fastener (where provided) until guidewire is removed.
- Seite 4 Warning: Ensure patency of each lumen of catheter prior to pressure injection to A pdf copy of this IFU is located at www.teleflex.com/IFU minimize the risk of catheter failure and/or patient complications. This is the “Arrow CVC” Summary of Safety and Clinical Performance (SSCP) 4.
- Seite 5 Date of Importer manufacture Teleflex, the Teleflex logo, Arrow, the Arrow logo and SharpsAway are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. © 2020 Teleflex Incorporated. All rights reserved.
- Seite 6 Centrální žilní katetr (CVC) s možností tlakových injekcí Indikace pro použití: 6. Prostup vodicího drátu do pravé strany srdce může vést k dysrytmiím, bloku pravého raménka Tawarova a perforaci Katetr Arrow je indikován k umožnění krátkodobého (< 30 dní) přístupu do centrálních cévy, stěny síně...
- Seite 7 4. Některé dezinfekční prostředky používané v místě zavedení Vytvořte počáteční přístup do žíly: katetru obsahují rozpouštědla, která mohou oslabit materiál Echogenická jehla (pokud je součástí balení): katetru. Alkohol, aceton a polyethylenglykol mohou oslabit Echogenická jehla se používá pro přístup k cévnímu systému pro zavedení vodicího drátu strukturu polyuretanových materiálů.
- Seite 8 Dokončete zavedení katetru: 11. Použijte centimetrové značky na vodicím drátu (pokud jsou součástí balení) jako referenci, která vám pomůže určit délku zavedení vodicího drátu. 21. Zkontrolujte průchodnost lumenu tak, že ke každé prodlužovací hadičce připojíte stříkačku a aspirujete, dokud neuvidíte volný tok žilní krve. POZNÁMKA: Jestliže vodicí...
- Seite 9 Datum výroby Dovozce Teleflex, logo Teleflex, Arrow, logo Arrow a SharpsAway jsou ochranné známky nebo registrované ochranné známky společnosti Teleflex Incorporated nebo jejích přidružených společností v USA a/nebo v dalších zemích. © 2020 Teleflex Incorporated. Všechna práva vyhrazena.
-
Seite 10: Indikationer For Brug
Centralt venekateter (CVK) til trykinjektion 7. Brug ikke for stor kraft ved anlæggelse eller fjernelse Indikationer for brug: af katetret eller guidewiren. For stor kraft kan føre til Arrow katetret er indiceret til at muliggøre kortvarig (< 30 dage) central veneadgang ved komponentskader eller brud. - Seite 11 kan også svække klæbeevnen mellem kateterfikseringen og så klinikeren kan identificere den nøjagtige position af kanylespidsen, når karret punkteres huden. under ultralyd. • Brug ikke acetone på katetrets overflade. • Brug ikke sprit til at væde katetrets overflade, og sørg Beskyttet kanyle/sikret kanyle (hvis medleveret): for at der ikke er sprit i et kateterlumen som et forsøg på...
- Seite 12 Afslut anlæggelse af katetret: BEMÆRK: Når der bruges en guidewire sammen med Arrow Raulerson sprøjten (helt aspireret) og en 6,35 cm (2-1/2 tommer) introducerkanyle, kan følgende 21. Kontroller åbenheden af lumen ved at påsætte en sprøjte på hver forlængerslange og referencepunkter anvendes til positionering: aspirere, indtil der ses frit gennemløb af veneblod.
- Seite 13 Anvendes inden Fabrikant tørt naturgummilatex steril barriere er beskadiget Fabrikationsdato Importør Teleflex, Teleflex-logoet, Arrow, Arrow-logoet og SharpsAway er varemærker eller registrerede varemærker tilhørende Teleflex Incorporated eller dets datterselskaber i USA og/eller andre lande. © 2020 Teleflex Incorporated. Alle rettigheder forbeholdes.
- Seite 14 Voor hogedrukinjectie geschikte centraal veneuze katheter (CVC) Indicaties voor gebruik: katheterisatie onder directe visualisatie uit te voeren om zo het risico van verstrikking van de voerdraad te beperken. De Arrow-katheter is geïndiceerd om kortdurend (< 30 dagen) centraal veneuze toegang 5.
-
Seite 15: Initiële Veneuze Toegang Verkrijgen
Voorzorgsmaatregelen: Voorzorgsmaatregel: Probeer naalden die al in de SharpsAway II- 1. Modificeer de katheter, de voerdraad of enige andere naaldenklembeker geplaatst zijn, daar niet weer uit te verwijderen. Deze component van de kit/set niet bij het inbrengen, gebruiken naalden zitten stevig vast. De naalden kunnen beschadigd raken als ze uit de of verwijderen. - Seite 16 Arrow GlideWheel Wire Advancer of Arrow Advancer (indien verstrekt): Waarschuwing: Bevestig de katheterklem en de bevestiger (indien verstrekt) De Arrow Advancer dient voor het rechtmaken van de “J”-tip van de voerdraad om deze in pas als de voerdraad verwijderd is. een Arrow Raulerson-spuit of een naald te kunnen inbrengen.
- Seite 17 Luer-aanzetstuk van de katheter om het risico van katheterfalen en/of Een pdf-bestand met deze gebruiksaanwijzing is beschikbaar op www.teleflex.com/IFU tipverplaatsing tot een minimum te beperken. Dit is de locatie van de samenvatting van de veiligheids- en klinische prestaties Waarschuwing: Staak hogedrukinjecties bij de eerste tekenen van extravasatie [Summary of Safety and Clinical Performance of SSCP] van de “Arrow CVC”...
- Seite 18 Productiedatum Importeur Teleflex, het Teleflex-logo, Arrow, het Arrow-logo en SharpsAway zijn handelsmerken of gedeponeerde handelsmerken van Teleflex Incorporated of verbonden ondernemingen in de VS en/of andere landen. © 2020 Teleflex Incorporated. Alle rechten voorbehouden.
-
Seite 19: Yleiset Varoitukset Ja Varotoimet
Paineinjektion kestävä keskuslaskimokatetri Käyttöaiheet: tai verisuonen, sydämen eteisen tai kammion seinämän puhkeaman. Arrow-katetri on tarkoitettu antamaan lyhytaikaisen (<30 päivää) keskuslaskimoyhteyden 7. Katetria tai ohjainvaijeria sijoitettaessa tai poistettaessa ei sellaisten sairauksien tai tilojen hoidossa, joissa tarvitaan keskuslaskimoyhteyttä, kuten saa käyttää liiallista voimaa. Liiallinen voima voi aiheuttaa mm. - Seite 20 4. Jotkin katetrin sisäänvientikohdassa käytetyt Ensimmäisen laskimoon pääsyn tekeminen: desinfiointiaineet sisältävät liuottimia, jotka voivat heikentää Kaikuinen neula (jos toimitettu): katetrimateriaalia. Alkoholi, asetoni ja polyeteeniglykoli Kaikuista neulaa käytetään verisuonistoon pääsyä varten, jotta ohjainvaijeri voidaan viedä voivat heikentää polyuretaanimateriaalien rakennetta. Nämä sisään katetrin asettamisen avuksi. Neulan kärki on tehostettu noin 1 cm:n matkalta, jotta aineet voivat myös heikentää...
- Seite 21 jotta ohjainvaijeria voidaan työntää eteenpäin (katso kuva 3A). Jatka, kunnes 20. Tarkista aina, että koko ohjainvaijeri on ehjä poistamisen jälkeen. ohjainvaijeri saavuttaa halutun syvyyden. Katetrin sisäänviennin loppuun suorittaminen: 11. Käytä ohjainvaijerin senttimetrimerkkejä (jos sellaisia on) apuna määritettäessä, 21. Tarkasta luumenin avoimuus liittämällä ruisku jokaiseen jatkoletkuun ja aspiroimalla, miten kauas ohjainvaijeri on työnnetty.
- Seite 22 Valmistus- Maahantuoja päivämäärä Teleflex, Teleflex-logo, Arrow, Arrow-logo ja SharpsAway ovat Teleflex Incorporated -yhtiön tai sen tytäryhtiöiden tavaramerkkejä tai rekisteröityjä tavaramerkkejä Yhdysvalloissa ja/tai muissa maissa. © 2020 Teleflex Incorporated. Kaikki oikeudet pidätetään.
-
Seite 23: Avertissements Et Précautions Généraux
Cathéter veineux central (CVC) pour injection sous pression 5. Ne pas utiliser une force excessive lors de l’introduction Indications : du guide ou du dilatateur de tissus car cela peut Le cathéter Arrow est indiqué pour assurer un accès veineux central à court terme (moins entraîner une perforation du vaisseau, un saignement ou de 30 jours) dans le traitement des maladies ou affections nécessitant un accès veineux l’endommagement d’un composant. - Seite 24 2. La procédure doit être pratiquée par un personnel • Si fourni, un système en mousse SharpsAway peut être utilisé pour y enfoncer les qualifié avec une excellente connaissance des points de aiguilles après utilisation. repères anatomiques, des techniques sécuritaires et des Précaution : Ne pas réutiliser les aiguilles une fois qu’elles ont été...
- Seite 25 10. Avancer le guide dans la seringue Raulerson Arrow d’ e nviron 10 cm jusqu’à ce qu’il REMARQUE : Les symboles des repères en centimètres sont visibles à partir de l’extrémité passe à travers les valves de la seringue ou dans l’aiguille de ponction. du cathéter.
-
Seite 26: Nettoyage Et Entretien
Avertissement : Arrêter les injections sous pression dès le premier signe d’une Une version PDF de cette notice d’utilisation est disponible sur www.teleflex.com/IFU extravasation ou d’une déformation du cathéter. Observer les protocoles et Voici l’adresse du résumé de sécurité et de performances cliniques du CVC Arrow après procédures de l’établissement concernant l’intervention médicale appropriée. - Seite 27 Teleflex, le logo Teleflex, Arrow, le logo Arrow et SharpsAway sont des marques commerciales ou des marques déposées de Teleflex Incorporated ou de ses sociétés affiliées, aux États-Unis et/ou dans d’autres pays. © 2020 Teleflex Incorporated. Tous droits réservés.
-
Seite 28: Zentraler Venenkatheter (Zvk) Für Druckinjektionen
Zentraler Venenkatheter (ZVK) für Druckinjektionen Indikationen: Kreislaufsystem hat, wird empfohlen, den Kathetereingriff unter direkter Sichtkontrolle durchzuführen, um das Risiko Der Arrow Katheter ist für einen kurzzeitigen (< 30 Tage) zentralvenösen Zugang zur zu vermindern, dass sich der Führungsdraht verfängt. Behandlung von Krankheiten oder Beschwerden, die einen zentralvenösen Zugang 5. -
Seite 29: Bei Der Infusion Von Medikamenten Mit Hohem
• • Thrombose Infektion an der 5. Kanüle entsorgen. • Austrittsstelle Unbeabsichtigte arterielle SharpsAway II Entsorgungsbehälter mit Sperrfunktion (sofern • Punktion Gefäßerosion enthalten): • • Verletzung von Nerven Falsche Lage der Zur Entsorgung von Kanülen wird der SharpsAway II Entsorgungsbehälter mit •... -
Seite 30: Führungsdraht Einbringen
• Das Spritzenventilsystem der Arrow Raulerson Spritze unter Verwendung der 13. Die Verweillänge unter Verwendung der Zentimetermarkierungen am Führungsdraht Druckübertragungssonde öffnen und auf pulsierenden Fluss prüfen. entsprechend der gewünschten Platzierungstiefe des Verweilkatheters anpassen. • Die Spritze von der Kanüle abnehmen und auf pulsierenden Fluss prüfen. 14. -
Seite 31: Sicherung Des Katheters
Komplikationen vertraut ist. 2. Das Lumen für die Druckinjektion identifizieren. Der Kurzbericht über Sicherheit und klinische Leistung (SSCP) zum Arrow ZVK steht nach dem 3. Die Durchgängigkeit des Katheters prüfen: Start der Europäischen Datenbank für Medizinprodukte/Eudamed unter folgender Adresse zur •... - Seite 32 Nummer beschädigt ist latex verwendet Herstellungs datum Importeur Teleflex, das Teleflex-Logo, Arrow, das Arrow-Logo und SharpsAway sind Marken oder eingetragene Marken von Teleflex Incorporated oder verbundenen Unternehmen in den USA und/oder anderen Ländern. © 2020 Teleflex Incorporated. Alle Rechte vorbehalten.
- Seite 33 Κεντρικός φλεβικός καθετήρας (CVC) για έγχυση υπό πίεση 5. Μην ασκείτε υπερβολική δύναμη κατά την εισαγωγή του Ενδείξεις χρήσης: οδηγού σύρματος ή του διαστολέα ιστού, καθώς αυτό μπορεί Ο καθετήρας Arrow ενδείκνυται για να επιτρέπει τη βραχυχρόνια (< 30 ημέρες) κεντρική να...
- Seite 34 Προφυλάξεις: • Μόλις τοποθετηθούν στο κύπελλο απόρριψης, οι βελόνες ασφαλίζουν αυτόματα στη θέση τους, έτσι ώστε να μην μπορούν να επαναχρησιμοποιηθούν. 1. Μην τροποποιείτε τον καθετήρα, το οδηγό σύρμα ή Προφύλαξη: Μην επιχειρήσετε να αφαιρέσετε τις βελόνες που έχουν οποιοδήποτε άλλο εξάρτημα του κιτ/σετ κατά τη διάρκεια τοποθετηθεί...
- Seite 35 Εισαγάγετε το οδηγό σύρμα: Προειδοποίηση: Μην κόβετε το οδηγό σύρμα για να αλλάξετε το μήκος του. Οδηγό σύρμα: Προειδοποίηση: Μην κόβετε το οδηγό σύρμα με νυστέρι. Διατίθενται κιτ/σετ με διάφορα οδηγά σύρματα. Τα οδηγά σύρματα διατίθενται σε διάφορες • Τοποθετήστε το κοπτικό άκρο του νυστεριού μακριά από το οδηγό σύρμα. διαμέτρους, μήκη...
- Seite 36 Μπορείτε να βρείτε ένα αντίγραφο αυτών των οδηγιών χρήσης σε μορφή αρχείου pdf στην • Εκπλύνετε σχολαστικά τον καθετήρα. ιστοσελίδα www.teleflex.com/IFU Προειδοποίηση: Βεβαιωθείτε για τη βατότητα όλων των αυλών του καθετήρα Αυτή είναι η τοποθεσία της Περίληψης των χαρακτηριστικών ασφάλειας και των κλινικών...
- Seite 37 κατασκευής Το Teleflex, το λογότυπο Teleflex, το Arrow, το λογότυπο Arrowκαι το SharpsAway είναι εμπορικά σήματα ή σήματα κατατεθέντα της Teleflex Incorporated ή συνδεδεμένων με αυτήν εταιρειών στις Η.Π.Α. ή/και σε άλλες χώρες. © 2020 Teleflex Incorporated. Με την επιφύλαξη παντός...
-
Seite 38: Avvertenze E Precauzioni Generali
Catetere venoso centrale (CVC) per iniezione a pressione Indicazioni per l’uso 5. Non esercitare una forza eccessiva nell’introdurre il filo guida o il dilatatore per tessuti, poiché così facendo si può Il catetere Arrow è stato concepito per consentire l’accesso venoso centrale a breve termine provocare la perforazione del vaso, sanguinamento o il (<30 giorni) per il trattamento di patologie o condizioni che richiedono l’accesso venoso danneggiamento del componente. - Seite 39 Precauzioni Precauzione – Non tentare di rimuovere gli aghi precedentemente inseriti nella 1. Non modificare il catetere, il filo guida o alcun altro coppetta di smaltimento con chiusura SharpsAway II. Questi aghi sono bloccati componente del kit/set durante l’inserimento, l’uso o la permanentemente in posizione.
- Seite 40 Dispositivo di avanzamento Arrow GlideWheel Wire Advancer o Avvertenza – Non lasciare in sede il dilatatore per tessuti come se fosse un dispositivo di avanzamento Arrow Advancer (se disponibile) catetere a permanenza. La permanenza in sede del dilatatore per tessuti espone Il dispositivo di avanzamento Arrow Advancer serve per raddrizzare la punta a “J”...
- Seite 41 Avvertenza – Per ridurre al minimo il rischio di malfunzionamento del catetere e/o di complicanze per il paziente, verificare la pervietà di ciascun lume del Nella pagina www.teleflex.com/IFU si può trovare una versione pdf delle presenti catetere prima dell’iniezione a pressione.
- Seite 42 Importatore fabbricazione Teleflex, il logo Teleflex, Arrow, il logo Arrow e SharpsAway sono marchi commerciali o marchi registrati di Teleflex Incorporated o delle sue società affiliate, negli USA e/o in altri Paesi. © 2020 Teleflex Incorporated. Tutti i diritti riservati.
- Seite 43 Trykkinjiserbart sentralt venekateter (SVK) 6. Innføring av ledevaieren i høyre del av hjertet kan medføre Indikasjoner for bruk: dysrytmier, høyre grenblokk og perforasjon av kar-, atrie- Arrow-katetret er indisert for å tillate kortvarig (< 30 dager) sentral venøs tilgang for eller ventrikkelvegg.
- Seite 44 svekke katetermaterialet. Alkohol, aceton og polyetylenglykol Beskyttet nål / sikkerhetsnål (hvis utstyrt): kan svekke strukturen i polyuretanmaterialene. Disse En beskyttet nål / sikkerhetsnål skal brukes i tråd med produsentens bruksanvisning. agensene også nedsette klistreevnen mellom kateterstabiliseringsanordningen og huden. Arrow Raulerson-sprøyte (hvis utstyrt): •...
- Seite 45 Forholdsregel: Ha et fast grep om ledevaieren til enhver tid. La tilstrekkelig Advarsel: Åpne skyveklemmen før infusjon gjennom lumen for å redusere ledevaierlengde stikke ut for håndtering. En ukontrollert ledevaier kan føre til risikoen for skade på forlengelsesslangen pga. for høyt trykk. vaieremboli.
- Seite 46 Brukes innen Produsent steril beskyttelse sollys naturgummilateks er skadet Produksjonsdato Importør Teleflex, Teleflex-logoen, Arrow, Arrow-logoen og SharpsAway er varemerker eller registrerte varemerker for Teleflex Incorporated eller tilknyttede selskaper, i USA og/eller andre land. © 2020 Teleflex Incorporated. Alle rettigheter forbeholdt.
- Seite 47 Cewnik do żył centralnych nadający się do wstrzykiwania pod ciśnieniem Wskazania: przy bezpośrednim uwidocznieniu, zapobiec uwięźnięciu prowadnika. Cewnik Arrow jest wskazany do umożliwiania krótkotrwałego (< 30 dni) dostępu do żył 5. Przy wprowadzaniu prowadnika lub rozszerzacza tkanek nie centralnych celem leczenia chorób lub schorzeń wymagających dostępu do żył centralnych, należy używać...
- Seite 48 Środki ostrożności: Środek ostrożności: Nie podejmować prób wyjęcia igieł umieszczonych w pojemniku na odpady SharpsAway II z blokadą. Igły te są zablokowane w 1. Podczas wprowadzania, używania i wyjmowania, nie wolno miejscu. Wyjmowanie igieł z pojemnika na odpady z użyciem siły może modyfikować...
- Seite 49 Arrow GlideWheel Wire Advancer lub Arrow Advancer (jeśli dostarczone): Ostrzeżenie: Zacisku ani elementu do mocowania cewnika (jeśli są dostępny) nie należy podłączać, dopóki nie zostanie wyjęty prowadnik. Przyrząd Arrow Advancer służy do prostowania końcówki „J” prowadnika w celu wprowadzenia prowadnika do igły lub strzykawki Arrow Raulerson. 18.
- Seite 50 Arrow International LLC: www.teleflex.com Środek ostrożności: Aby zminimalizować ryzyko awarii cewnika i/lub Wersja pdf niniejszej instrukcji użycia znajduje się pod adresem www.teleflex.com/IFU przemieszczenia końcówki, nie wolno przekraczać dziesięciu (10) iniekcji ani wartości maksymalnej zalecanej prędkości przepływu, umieszczonej w Adres podsumowania dotyczącego bezpieczeństwa i skuteczności klinicznej (SSCP)
- Seite 51 Data produkcji Importer Teleflex, logo Teleflex, Arrow, logo Arrow i SharpsAway są znakami towarowymi lub zastrzeżonymi znakami towarowymi firmy Teleflex Incorporated lub jej spółek stowarzyszonych w Stanach Zjednoczonych i/lub w innych krajach. © 2020 Teleflex Incorporated. Wszelkie prawa zastrzeżone.
-
Seite 52: Indicações De Utilização
Cateter Venoso Central (CVC) para Injeção Pressurizada Indicações de utilização: 6. A passagem do fio-guia para dentro do coração direito pode causar disritmias, bloqueio do feixe nervoso direito O cateter Arrow está indicado para permitir o acesso venoso central a curto prazo e perfuração da parede de um vaso, da aurícula ou do (<... - Seite 53 3. Use as precauções padrão e siga as políticas institucionais Prepare o cateter: relativas a todos os procedimentos, incluindo a eliminação 6. Irrigue cada lúmen com soro fisiológico estéril normal para injeção, para estabelecer a segura dos dispositivos. permeabilidade e purgar o(s) lúmen(es). 4.
- Seite 54 • numerais: 5, 15, 25, etc. 10. Avance o fio-guia na seringa Arrow Raulerson aproximadamente 10 cm até passar pelas válvulas da seringa ou entrar na agulha introdutora. • bandas: cada banda denota um intervalo de 10 cm, onde uma banda indica •...
-
Seite 55: Cuidados E Manutenção
Advertência: Interrompa as injeções pressurizadas ao primeiro sinal de Em www.teleflex.com/IFU, encontra-se uma cópia destas instruções de utilização em extravasamento ou deformação do cateter. Siga as políticas e os procedimentos formato PDF. - Seite 56 Data de fabrico Importador Teleflex, o logótipo Teleflex, Arrow, o logótipo Arrow e SharpsAway são marcas comerciais ou marcas comerciais registadas da Teleflex Incorporated ou das respetivas filiais nos EUA e/ou em outros países. © 2020 Teleflex Incorporated. Reservados todos os direitos.
- Seite 57 Tlakový vstrekovací centrálny žilový katéter (CŽK) 7. Pri zavádzaní alebo vyťahovaní katétra alebo vodiaceho Indikácie na použitie: drôtu nevyvíjajte nadmernú silu. Nadmerná sila môže Katéter Arrow je určený na umožnenie krátkodobého (< 30 dní) centrálneho žilového spôsobiť poškodenie alebo zlomenie komponentu. Ak prístupu pri liečbe chorôb alebo stavov, ktoré...
- Seite 58 4. Niektoré dezinfekčné prostriedky použité na mieste zavedenia Získajte úvodný prístup do žily: katétra obsahujú rozpúšťadlá, ktoré môžu oslabiť materiál Echogénna ihla (ak je poskytnutá): katétra. Alkohol, acetón a polyetylénglykol môžu oslabiť Echogénna ihla sa používa na zabezpečenie prístupu do cievnej sústavy na zavedenie štruktúru polyuretánových materiálov.
- Seite 59 • Ak používate štandardný zavádzač Arrow Advancer, zdvihnite palec a • V tejto situácii môže spätné potiahnutie za vodiaci drôt viesť k pôsobeniu neprimeranej sily vedúcej k zlomeniu vodiaceho drôtu. zavádzač Arrow Advancer potiahnite približne 4 – 8 cm smerom od striekačky Arrow Raulerson alebo zavádzacej ihly.
- Seite 60 Dátum výroby Dovozca Teleflex, logo Teleflex, Arrow, logo Arrow a SharpsAway sú ochranné známky alebo registrované ochranné známky spoločnosti Teleflex Incorporated alebo jej spriaznených subjektov v USA alebo iných krajinách. © 2020 Teleflex Incorporated. Všetky práva vyhradené.
-
Seite 61: Advertencias Y Precauciones Generales
Catéter venoso central (CVC) para inyección a presión 6. El paso de la guía a la parte derecha del corazón puede causar Indicaciones de uso: arritmias, bloqueo de la rama derecha del haz y perforación El catéter Arrow está indicado para permitir el acceso venoso central a corto plazo de la pared vascular, auricular o ventricular. - Seite 62 3. Tome las precauciones habituales y siga las normas del Prepare el catéter: centro para todos los procedimientos, incluida la eliminación 6. Lave cada luz con una solución salina normal estéril para inyección con el fin de segura de los dispositivos. establecer la permeabilidad y cebar las luces.
- Seite 63 • El avance de la guía a través de la jeringa Raulerson de Arrow puede requerir un 19. Sujete el catéter a la profundidad deseada y extraiga la guía. suave movimiento de giro. Precaución: Si se encuentra resistencia al intentar extraer la guía después de •...
- Seite 64 Realice la intervención médica apropiada según las sitio web de Arrow International LLC: www.teleflex.com normas y los procedimientos del centro. Para obtener un ejemplar de estas instrucciones de uso en pdf, vaya a Precaución: Caliente el medio de contraste a la temperatura corporal antes de...
- Seite 65 Teleflex, el logotipo de Teleflex, Arrow, el logotipo de Arrow y SharpsAway son marcas comerciales o registradas de Teleflex Incorporated o de sus filiales, en EE. UU. o en otros países. © 2020 Teleflex Incorporated. Reservados todos los derechos.
-
Seite 66: Indikationer För Användning
Tryckinjicerbar central venkateter (CVK) 6. Införing av ledaren i hjärtats högra del kan orsaka Indikationer för användning: rytmrubbningar, högersidigt skänkelblock och perforation Arrow-katetern är indicerad för att tillhandahålla kortvarig (<30 dagar) central av kärlväggen, förmaksväggen eller kammarväggen. venåtkomst för behandling av sjukdomar eller tillstånd som kräver central venåtkomst, 7. - Seite 67 4. Vissa desinfektionsmedel som används vid kateterns Skapa inledande venåtkomst: införingsställe innehåller lösningsmedel som kan försvaga Ekogen nål (i förekommande fall): katetermaterialet. Alkohol, aceton och polyetylenglykol En ekogen nål används för att möjliggöra åtkomst till kärlsystemet för införing av en ledare försvaga strukturen polyuretanmaterial.
- Seite 68 Slutföra införingen av katetern: 11. Använd centimetermarkeringarna (i förekommande fall) på ledaren som referens för att lättare kunna fastställa hur stor del av ledaren som har förts in. 21. Kontrollera lumens öppenhet genom att ansluta en spruta till varje förlängningsslang OBS! Om ledaren används i kombination med Arrow Raulerson-sprutan (helt aspirerad) och aspirera tills ett fritt flöde av venblod observeras.
- Seite 69 Tillverknings- Importör datum Teleflex, Teleflex-logotypen, Arrow, Arrow-logotypen och SharpsAway är varumärken eller registrerade varumärken som tillhör Teleflex Incorporated eller dess dotterbolag i USA och/eller i andra länder. © 2020 Teleflex Incorporated. Med ensamrätt.
- Seite 72 EU Authorized Representative Arrow International LLC and Importer: Subsidiary of Teleflex Incorporated Teleflex Medical 3015 Carrington Mill Blvd., Morrisville, NC 27560 USA IDA Business and Technology Park USA: 1 866 246 6990 | International: +1 919 544 8000 Dublin Road, Athlone, Co. Westmeath, Ireland...